A NEW DAWN FOR FIGHT AGAINST MALARIA IN THE AFRICAN REGION
Bartholomew Dicky Akanmori1, Joseph Okeibunor1*, and Matshidiso Rebecca Moeti2
1WHO Regional Office for Africa, Brazzaville, Congo
2WHO Regional Director for Africa, Brazzaville, Congo
Abstract
The WHO Regional Office for Africa is systematically taking steps to realize the goals of malaria control in the Region. One of such steps is the pursuit for the development of malaria vaccine, which is being tested in three countries in the Region, namely Ghana, Kenya and Malawi. This paper reviews the potential contributions of a vaccine against malaria in endemic regions like sub-Saharan Africa beyond just coming as another intervention for malaria control. The injectable vaccine, RTS,S, was developed to protect young children from the most deadly form of malaria, Plasmodium falciparum, which is endemic in the Region. However, sceptics argued that this could be unlikely outside of rigorously controlled clinical trials, as well as waning efficacy over time. There has been calls for cautious optimism and emphasized that “the vaccine is just an additional tool in the current limited armamentarium for making progress against malaria”. This review demonstrates the benefits of having the malaria vaccine are numerous, including strengthening national immunization and malaria control programmes; stimulating and boosting the scale-up of the existing interventions which have so far made significant reductions in malaria burden across several countries of the region.
Introduction
Malaria still kills hundreds of thousands of people each year, despite huge public and private investments to promote antimalarial commodities globally. Most affected are children and pregnant women in the poorest countries especially those in sub-Saharan Africa1–6. However, in the last decade and a half, global malaria mortalities and morbidity dropped by 45% and 25% respectively following the expanded funding for malaria control interventions like long-lasting insecticidal nets, indoor residual spraying programmes and access to artemisinin combination therapy1. The number of people who become sick or die from malaria fell by 33% and 25% in the hardest hit regions like sub-Saharan Africa7. Nevertheless, malaria remains one of the most salient public health concerns in the world today7–9. In 2016, 91 countries reported a total of 216 million cases of malaria, an increase of 5 million cases over the 212 million cases reported in 2015 and the global tally of malaria deaths reached 445 000 deaths10. World Malaria Day, April 24th 2017 and its theme “Malaria prevention: let’s close the gap” remind the global community of the need to intensify the fight against malaria with the deployment of new and more efficient tools and tactics, especially in endemic regions like sub Saharan Africa.
One major challenge has been the absence of a licensed vaccine against malaria. According to Ouattara and Laurens, a malaria vaccine is viewed in many quarters as a necessary tool in stepping up the fight against malaria9. The World Health Organization (WHO) has also set an agenda to license malaria vaccine by 2030 that target Plasmodium falciparium and Plasmodium vivax. This paper briefly reviews the importance of a vaccine against malaria in endemic regions like sub-Saharan Africa and steps being put in place by the WHO Regional Office for Africa to realize the goals of this agenda. The injectable vaccine, RTS,S, was developed to protect young children from the most deadly form of malaria, Plasmodium falciparum. RTS,S will be assessed in the pilot programmes as a complementary malaria control tool that could be added to the core package of WHO-recommended measures for malaria prevention. WHO and partners will support the countries to pilot the current vaccine and after SAGE recommendations will further support wider vaccine implementation across endemic areas while supporting R&D for development of the next generation of more effective malaria vaccines. The target product profile for the next generation of malaria vaccines has been set by WHO11 and scientists, sponsors and vaccine developers are vigorously working to develop more effective vaccines against the disease with WHO and partner support.
WHO malaria vaccine pilot programme in the African Region
During the 2017 World Malaria Day celebration the World Health Organization Regional Office for Africa (WHO/AFRO) announced the participation of three African countries, namely Ghana, Kenya, and Malawi in a WHO-coordinated pilot implementation programme that will make the world’s first malaria vaccine available in selected areas, beginning in 2018. Although this is far from the ideal vaccine, RTS,S has the potential to improve malaria control through prevention of cases and deaths.
The WHO Regional Office for Africa therefore views the prospect of a malaria vaccine as great news and great relief given the burden of the disease in the Region. Africa bears the greatest burden of malaria worldwide. Global efforts in the last 16 years have led to a 62 percent reduction in malaria deaths between 2000 and 2016, yet approximately 445,000 people died of the disease in 2016 globally, the majority of them (407,000 or approximately 91%) young persons in the WHO African Region. Both Table 1 and Figure 1 show clearly the level of endemicity of malaria and the burden of malaria cases in the African Region respectively, compared to other Regions of the World Health Organization. The pilot will ensure informed decisions on the wider use of this vaccine. The WHO pilot programme will assess whether the vaccine’s protective effect in children aged five months to seventeen months old during Phase III testing, can be replicated in real-life. Specifically, the pilot will assess the feasibility of delivering the required four doses of RTS,S, the vaccine’s potential role in reducing childhood deaths, and its safety in the context of routine use.
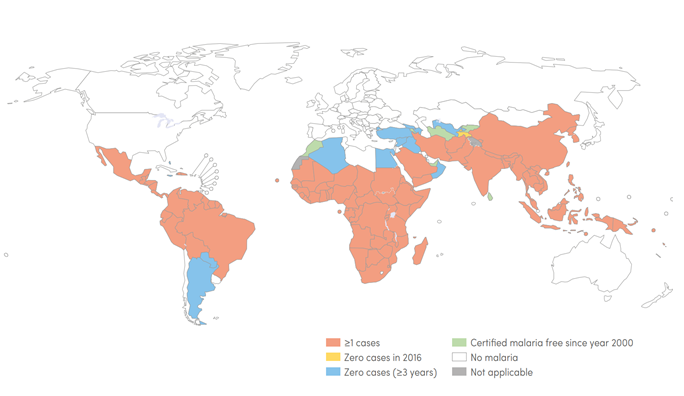
Figure 1. Global Malaria Endemicity 2000 and 2016
Source: World Malaria Report 2017
Legend: The map shows countries and territories with indigenous cases in 2000 and their status by 2016. All countries in the WHO European Region reported zero indigenous cases in 2016 unlike countries in African Region, where such countries are uncommon.
Table 1: Estimated malaria cases by WHO Region, 2016
| Range | Number of cases (000's) | |||||
|---|---|---|---|---|---|---|
| AFR | AMR | EMR | SEAR | WPR | Global | |
| Estimated Total | 194000 | 875 | 4300 | 14600 | 1600 | 216000 |
| Estimated P. vivax | 859 | 556 | 1790 | 4960 | 385 | 8550 |
| % cases P. Vivax | 10.4 | 64 | 42 | 34 | 24 | 4 |
Source: World Malaria Report. 2017
Legend: The map shows countries and territories with indigenous cases in 2000 and their status by 2016. All countries in the WHO European Region reported zero indigenous cases in 2016 unlike countries in African Region, where such countries are uncommon.
RTS, S was developed by Glaxo-Smith-Kline (GSK) and is the first malaria vaccine to have successfully completed a Phase III clinical trial. The trial was conducted between 2009 and 2014 through a partnership involving GSK, the PATH Malaria Vaccine Initiative (with support from the Bill & Melinda Gates Foundation), and a network of African research sites in seven African countries—including Ghana, Kenya, and Malawi. It is also the first malaria vaccine to have obtained a positive scientific opinion from a stringent medicines regulatory authority, the European Medicines Agency (EMA), which approved RTS,S in July 2015.
In October 2015, two independent WHO advisory groups, comprised of the world’s foremost experts on vaccines and malaria, recommended pilot implementation of RTS,S in three to five settings in sub-Saharan Africa. The recommendation came from the Strategic Advisory Group of Experts (SAGE) on Immunization and the Malaria Policy Advisory Committee (MPAC), following a joint review of all available evidence on the vaccine’s safety, and efficacy. The WHO formally adopted the recommendation in January 2016.
While the prospect of availability and roll out of the RTS,S vaccine is a remarkable achievement, critics have pointed out several serious shortcomings, including the intensive regimen (three injected doses at months 0, 1, and 2, and a booster dose at month 20)12. This they argued could be unfeasible outside of rigorously controlled clinical trials, as well as waning efficacy over time. Based on this, the Lancet called for cautious optimism and emphasized that “the vaccine is just an additional tool in the current limited armamentarium for making progress against malaria”.
It is however, important at this point to reiterate the benefits of having the malaria vaccine. One, rather than just complement other interventions, the vaccine, if successfully piloted in the three countries will further strengthen national immunization and malaria control programmes, stimulate and boost the scale-up of the existing interventions which have so far made significant reductions in malaria burden across several countries of the region. For instance these pilots serve as an opportunity for countries to promote the use of ITNs, improve malaria surveillance and case detection as well as provide better case management of malaria. The phase III clinical trial was carried out in areas with very high ITN coverage and it is expected that ITN coverage will similarly be improved with vaccine delivery and consistent with the SAGE recommendations. Effective communication about malaria treatment and prevention can be undertaken as part of vaccine implementation. The training which will precede the implementation of the malaria vaccine will undoubtedly complement existing capacity to deliver other interventions, while the vaccination will serve as a vehicle for the delivery of other childhood interventions as exemplified by the polio plus campaigns13–15. There will also be improvements in data collection, review, analysis and utilization capacities. Other opportunities which could leverage delivery of existing interventions include improvements in logistics, the diagnosis and management of cases of malaria which may be presented at immunization sessions.
Public health innovations can improve the delivery of existing interventions in general16. Voluntary Medical Male Circumcision Programs have improved HIV testing and counseling uptake as well as enrollment for anti-retroviral therapy (ART) among young men17. It is therefore conceivable that the malaria vaccine could make a more significant impact than anticipated through an increase in coverage with existing potent antimalarial interventions and consequently avert many more cases of malaria and to save more lives in the region.
References
- World Health Organization. World malaria report 2013. World Health 2013; WHO/HTM/GM: 238.
- World Health Organization. World Malaria Report 2010. World Heal Organ 2010; : 1–137.
- Heggenhougen HK, Hackethal V, Vivek P. The behavioural and social aspects of malaria and its control. CdrwwwWhoInt. 2003; 214.
- Barbara S. Malaria continues to threaten pregnant women and children. Popul Ref Bur Artic 2001. http://www.prb.org/Publications/Articles/2001/MalariaContinuestoThreatenPregnantWomenandChildren.aspx.
- Steketee RW, Nahlen BL, Parise ME, et al. The burden of malaria in pregnancy in malaria-endemic areas. In: American Journal of Tropical Medicine and Hygiene. 2001; 28–35.
- Desai M, ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007; 7: 93–104.
- Slutsker L, Kachur SP. It is time to rethink tactics in the fight against malaria. Malar J. 2013; 12: 140.
- Okeibunor JC, Orji BC, Brieger W, et al. Preventing malaria in pregnancy through community-directed interventions: evidence from Akwa Ibom State, Nigeria. Malar J. 2011; 10: 227.
- Ouattara A, Laurens MB. Vaccines against malaria. Clin Infect Dis. 2015; 60: 930–6.
- World Health Organization. World Malaria Report 2016. Geneva, 2016 http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/.
- Vannice KS, Brown G V., Akanmori BD, et al. MALVAC 2012 scientific forum: Accelerating development of second-generation malaria vaccines. Malar J. 2012; 11. DOI:10.1186/1475-2875-11-372.
- Lancet T. The next chapter in malaria eradication. Lancet. 2017; 389: 1772.
- Anand A, Luman ET, O’Connor PM. Building on success - potential to improve coverage of multiple health interventions through integrated delivery with routine childhood vaccination. J Infect Dis. 2012; 205: S28–39.
- Clements CJ, Nshimirimanda D, Gasasira A. Using immunization delivery strategies to accelerate progress in Africa towards achieving the Millennium Development Goals. Vaccine. 2008; 26: 1926–33.
- Mihigo R, Anya B, Okeibunor J, et al. African vaccination week as a vehicle for integrated health service delivery. BMC Health Serv Res 2015; 15. DOI:10.1186/s12913-015-0989-7.
- Rogers EM. Diffusion of Innovations, 5th Edition. 2003.
- Kikaya Virgile, Skolnik Laura, Garcia Macarena C, et al. Voluntary medical male circumcision programs can address low HIV testing and counseling usage and ART enrollment among young men: Lessons from Lesotho. PLoS One. 2014; 9. http://journals.plos.org/plosone/article/comments?id=10.1371/journal.pone.0083614.
