Metastatic Carcinoma to the Testis - A Mini Review
Gang Wang*
Department of Pathology, BC Cancer Vancouver Centre, University of British Columbia, Vancouver, BC, Canada
Abstract
Metastatic carcinomas to the testis are extremely rare and have been reported only in autopsy series or case reports. However, when they occur, the metastatic tumors in the testis are usually unilateral and solitary, may have overlap growth patterns and cytological features with primary testicular tumors, including those of rete/epididymis origin, mesothelial origin and Sertoli cell tumor. It will make the diagnosis very challenging, especially when there is no known history of a primary tumor in other sites. Metastatic prostatic adenocarcinoma counts nearly half of the overall metastatic carcinoma in the testis, followed by colorectal carcinoma and renal cell carcinoma. Here, we review our experience and summarize the reported cases from the literature, to emphasize some of the unusual aspects of metastatic carcinoma to the testis, and discuss the main differential diagnoses for this rare condition. Awareness of the features of these tumors, consideration of the possibility of metastasis and appropriate ancillary studies are the keys to the accurate diagnosis of these cases.
Introduction
Metastatic carcinomas to the testis are extremely rare and are most commonly incidental findings at autopsy in patients who have died of cancer1. The testes are regarded as a ‘tumor sanctuary’, as the relatively low temperature of the scrotum may provide unacceptable conditions for the establishment of metastatic tumor cells2. Additionally, the presence of the blood-testis barrier formed by Sertoli cells, which physiologically aims to protect spermatozoa, may also play an indirect role in the prevention of testicular metastasis3. Nonetheless, cancer metastasis to the testis does occur and may simulate primary testicular neoplasms, making the diagnosis challenging. Here, we review our experience and summarize the reported cases in the literature, to emphasize some of the unusual aspects of metastatic carcinoma to the testis.
Metastatic prostatic carcinoma
Prostatic adenocarcinoma counts nearly half of the overall metastatic carcinoma in the testis. At the time of orchiectomy, majority of the patients have a known history of prostatic carcinoma or are known to have an elevated serum prostate-specific antigen (PSA) level. On gross examination, most of the cases show distinct nodules or large masses which are yellow-white or tan, firm to fleshy, with circumscribed borders. Occasionally it can show diffuse involvement of the testis with no distinct masses4-6.
The most characteristic feature of the metastatic prostatic adenocarcinoma in the testis is the prominent involvement of the tubular system (Figure 1a). The metastatic tumor maintains the classic histologic and cytologic features of prostate cancer. However, almost all the metastatic tumors show Gleason pattern 4 or 5. Cribriform pattern is most frequently seen (Figure 1b) but solid growth pattern is common as well (Figure 1c). Sometimes, the tumor shows the appearance of ductal type, consisting of tall columnar cells with prominent nucleoli either radially arranged on fibrovascular cores or having an intraluminal cribriform arrangement (Figure 1d). Additional patterns such as fused glands, trabeculae, cords, and single cells can also be seen. Lymphovascular invasion, mostly at the periphery of the main tumor, is often identified. Ancillary tests can help to make a correct diagnosis. Metastatic prostate cancer would be positive for PSA (Figure 1e), but can be really focal or even negative. Other markers such as NKX3.1 or ERG (Figure 1f) would be helpful in this scenario.
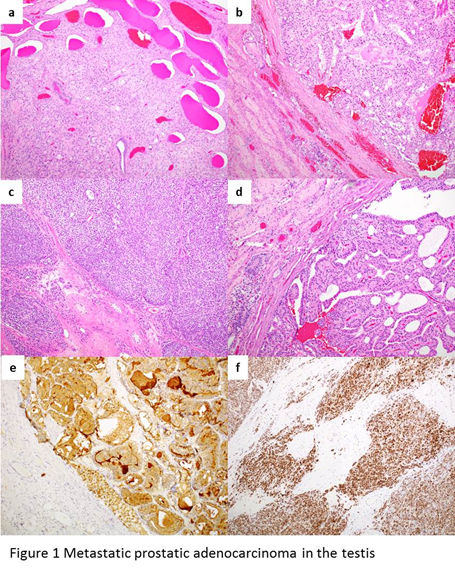
Figure 1: a, the most characteristic feature of the metastatic prostatic adenocarcinoma in the testis is the prominent involvement of the tubular system. b, the metastatic tumor shows cribriform pattern. c, the metastatic tumor shows solid growth pattern. d, some tumor shows the appearance of ductal type, consisting of tall columnar cells with prominent nucleoli either radially arranged on fibrovascular cores or having an intraluminal cribriform arrangement. e, metastatic prostate cancer is positive for PSA. f, metastatic prostate cancer is positive for ERG.
Metastatic renal cell carcinoma
Metastatic renal cell carcinoma (RCC) involving the testis comprises a small portion in this group of cases. Most of the cases (including the reported in the literature and our unpublished data) involve unilateral testis, but bilateral involvement of RCC in the testis has also been reported. There is slight tendency for the metastasis to occur in the ipsilateral testis, which may be related to preferential routes of spread between the kidney and the testis, including retrograde venous spread via the spermatic vein7-9.
Most of the reported metastatic RCCs in the testis are clear cell type2,3,7-20. On low-power view, the tumor involves the underlying testicular parenchyma and had broad pushing borders (Figure 2a). Most of the tumors show mixed patterns of hollow tubules, cysts, and alveoli or well-vascularized nests (Figure 2b). At high-power magnification, the polygonal tumor cells have lightly eosinophilic to abundant clear cytoplasm. The cystic space contains macrophages and proteinaceous eosinophilic fluid (Figure 2c). The WHO/ISUP grades of the tumors range from grade 2 to sarcomatoid dedifferentiation (WHO/ISUP grade 4). Lymphovascular invasion is often noted (Figure 2d). Residual testicular parenchyma show decreased spermatogenesis, tubular atrophy, and fibrosis, with unremarkable interstitial Leydig cells. By immunohistochemistry, the metastatic RCC cells are positive for pan-keratin, PAX8 (Figure 2e), CD10, and CAIX (Figure 2f). There is variable positivity for vimentin. The CK7 immunostain is negative in the tumor cells.
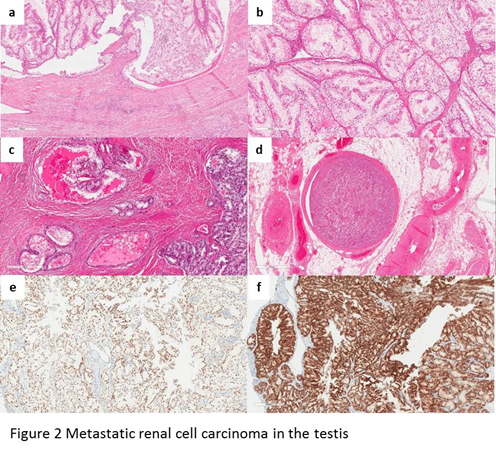
Figure 2: a, at low-power magnification, the metastatic RCC shows broad pushing border; b, metastatic RCC has mixed patterns of hollow tubules, cysts, and alveoli, or well-vascularized nests; c, the cysts are lined by clear cells and proteinaceous eosinophilic fluid; d, lymphovascular invasion is noted; e, the cells of metastatic RCC are positive for PAX8; f, the cells of metastatic RCC are positive for CAIX.
Metastatic colorectal carcinoma
The metastatic colorectal carcinoma to the testis is less common than those from the prostate or kidney. Most of the cases are unilateral testicular masses, might have not known primary colonic adenocarcinoma at the time of presentation4,21-23. On gross examination, the tumors are usually gray-white to tan-pink solitary masses with necrosis and hemorrhage.
Most of the tumors show a multinodular pattern (Figure 3a). Characteristically there are well-developed foci of cribriform glands lined by tall columnar cells with densely eosinophilic cytoplasm. “Dirty” necrosis or large cystic spaces containing mucinous secretion often present in the lumens of the neoplastic glands (Figure 3b). Lymphovascular invasions may be identified. Typically the tumor cells would be positive for CDX2 and cytokeratin 20, and negative for cytokeratin 7.
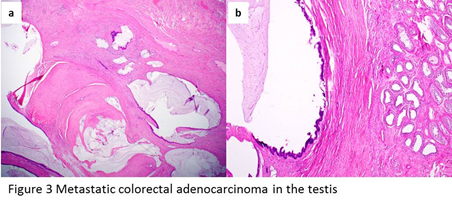
Figure 3: a, metastatic colonic adenocarcinoma shows a multinodular pattern. b, large cystic spaces lined by tall columnar cells with densely eosinophilic cytoplasm, containing mucinous secretion in the lumens of the neoplastic glands.
Metastatic squamous cell carcinoma from non-lung primaries
Most of the metastatic squamous cell carcinomas to the testis are from skin and they show similar morphology as in the primary site. Most of the tumors are well-circumscribed, composed of irregularly infiltrating and anastomosing nests of large cells with vesicular chromatin, large nucleoli, and abundant cytoplasm (Figure 4a). Quite often, the center of the mass is filled with gray-colored cheesy keratinous materials. Immunohistochemistry is positive for squamous cell carcinoma markers such as p40 (Figure 4b), cytokeratin 5/6 and p63.
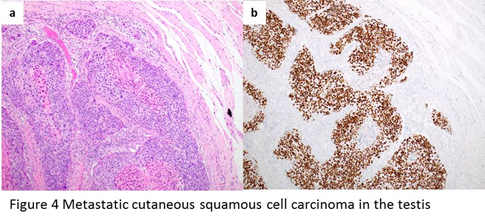
Figure 4: a, metastatic cutaneous squamous cell carcinoma shows well-circumscribed, composed of irregularly infiltrating and anastomosing nests of large cells with vesicular chromatin, large nucleoli, and abundant cytoplasm. The center of the mass is filled with gray-colored cheesy keratinous materials. b, immunohistochemistry shows positive stain of p40 for metastatic squamous cell carcinoma cells.
Metastatic lung carcinoma
Both adenocarcinoma and squamous cell carcinoma of the lung have been reported metastatic to the testis24-27. On gross examination, the tumors are usually solitary gray-tan intraparenchymal masses. On microscopic examination, adenocarcinoma involves the intertubular spaces of the testis, with papillary, micropapillary, acinar, mucinous or poorly differentiated patterns. Immunostains for TTF-1 highlight the tumor cell nuclei. Metastatic lung squamous cell carcinoma would show nests and islands of malignant squamous epithelium in a desmoplastic stroma that effaced the seminiferous tubules. Lymphovascular invasion can be identified.
Metastatic urothelial carcinoma
Metastatic urothelial carcinoma to the testis usually occurs in elderly patients, often presents as testicular enlargement with or without pain and swelling. Most of the patients have a prior history of urothelial carcinoma of the bladder or renal pelvis, but some patients are only subsequently found to have primary urothelial carcinoma4,28-30. On gross examination, the tumors are usually solitary, tan-red and tan-yellow nodules, or can be no discrete mass, but the cut surface of the testis would be abnormally firm and yellow, and may have foci of hemorrhage.
On microscopic examination, the tumors show variably sized, solid nests and larger, anastomosing islands of malignant epithelial cells with pale to eosinophilic cytoplasm. The tumor cells have predominantly intertubular pattern but occasionally completely effacing the testicular parenchyma4. Lymphovascular invasion could be easily appreciated.
Differential diagnosis
The diagnosis of metastatic carcinoma should not be difficult once the pathologists are aware of this rare scenario. A previous history would be helpful, although it is not always known. Here we discuss some of the main differential diagnoses for metastatic carcinoma in the testis.
Primary testicular germ cell tumors
Germ cell tumors, especially embryonal carcinoma and yolk sac tumor can show glandular growth pattern, resembling adenocarcinoma. However, the patients of germ cell tumor are typically in 30’s and 40’s, much younger than the patients with metastatic carcinoma to the testis (usually older than 50 year-old). Embryonal carcinoma often mixed with other growth patterns, such as solid and papillary patterns, with highly pleomorphic tumor cells, coarse or vesicular chromatin, prominent and irregular nucleoli. The tumor cells are typically positive for CD30, OCT3/4, PLAP and SALL431,32. Yolk sac tumor also frequently has multiple growth patterns, with microcystic or reticular patterns most frequent. The tumor cells are positive for glypican-3, AFP and SALL432,33. As components of germ cell tumor, embryonal carcinoma and yolk sac tumor usually show a background of germ cell neoplasm in situ. It should be aware that carcinoma can arise from preexisting teratoma or other germ cell tumors, so called somatic transformation34,35. It is more commonly in retroperitoneal lymph nodes or other metastatic sites, but can also within the primary testicular tumors. Most of time, it is in the background of more mature teratoma and germ cell neoplasm in situ.
Tumors of mesothelial origin
Adenomatoid tumor is a benign tumor of mesothelial cell origin, which has a variety of growth patterns, including glands, cysts, tubules, cords or isolated cells. It can mimic an adenocarcinoma. Grossly, it is usually a small, well-circumscribed, white-tan, homogenous, firm adnexal mass. The tumor cells are cuboidal to flat or ovoid with vacuoles, and minimal cytological atypia, and are positive for calretinin and WT1, which confirm the mesothelial origin36-38. Malignant mesothelioma arises from mesothelial cells of tunica vaginalis. It can grow with various patterns, including papillary, tubulopapillary, glandular or solid, and may be difficult to be distinguished from adenocarcinoma. The tumor cells are typically positive for mesothelial markers, such as calretinin, CK5/6, D2-40 and WT139-41. It should be noted that tumors of mesothelial origin express CAIX which can confound the diagnosis of metastatic clear cell renal cell carcinoma42.
Adenocarcinoma of the rete testis or epididymis
Adenocarcinoma of the rete testis or epididymis is extremely rare. It has been reported in patients with a wide age range, but most of the patients are older than 60, which overlap the age range of metastatic carcinoma to the testis. Quite often it is a diagnosis of exclusion. Some helpful features to differentiate it from metastatic adenocarcinoma are the morphologic transition from non-neoplastic rete or epididymis to adenocarcinoma (including in situ changes), and positivity for PAX841,43.
Primary testicular tumors mimicking renal cell carcinoma
In primary testicular tumors, some morphologically resemble RCC. Sertoli or Sertoli-Leydig tumor can show solid nests and tubular patterns as in clear-cell RCC. However, they lack the intricate and delicate vascular network, instead thick-walled and sometimes hyalinized vessels may be prominent. Immunostains for inhibin are typically positive in Sertoli cell tumors44,45, while PAX8 immunostain is positive in RCC. Classic seminoma, a common primary testicular tumor, may present with prominence of clear cells as in metastatic RCC. However, seminoma patients are typically younger (mean age, 40 years) than those with metastatic RCC (mean age, 64 years). Furthermore, seminoma lacks the typical vasculature of clear cell RCC, instead it usually demonstrates a conspicuous lymphocytic infiltrate, prominent stromal component, a background of germ cell neoplasia in-situ, as well as the immunohistochemical positivity for PLAP46,47.
In summary, without known history of a primary tumor, cases of metastatic carcinoma to the testis would be a great diagnostic challenge, because they are usually solitary and unilateral, and may have overlap growth patterns and cytological features with primary tumors, including those of rete/epididymis origin, mesothelial origin and Sertoli cell tumor. Awareness of the features of these tumors, consideration of the possibility of metastasis and appropriate ancillary studies are the keys to the accurate diagnosis of these cases.
References
-
- Garcia-Gonzalez R, Pinto J, Val-Bernal JF. Testicular metastases from solid tumors: an autopsy study. Annals of diagnostic pathology. 2000; 4(2): 59-64.
- Camerini A, Tartarelli G, Martini L, et al. Ipsilateral right testicular metastasis from renal cell carcinoma in a responder patient to interleukine-2 treatment. International journal of urology : official journal of the Japanese Urological Association. 2007; 14(3): 259-260.
- Dell'Atti L. Unusual Ultrasound Presentation of Testicular Metastasis from Renal Clear Cell Carcinoma. Rare tumors. 2016; 8(3): 6471.
- Ulbright TM, Young RH. Metastatic carcinoma to the testis: a clinicopathologic analysis of 26 nonincidental cases with emphasis on deceptive features. The American journal of surgical pathology. 2008; 32(11): 1683-1693.
- Blacklock AR. Testicular metastasis from carcinoma of the prostate. British journal of urology. 1984; 56(2): 221-222.
- Bradham AC. Prostatic carcinoma with metastasis to the testicle. The Journal of urology. 1951; 66(1): 122-126.
- Dieckmann KP, Due W, Loy V. Intrascrotal metastasis of renal cell carcinoma. Case reports and review of the literature. European urology. 1988; 15(3-4): 297-301.
- Hanash KA, Carney JA, Kelalis PP. Metastatic tumors to testicles: routes of metastasis. The Journal of urology. 1969; 102(4): 465-468.
- Pienkos EJ, Jablokow VR. Secondary testicular tumors. Cancer. 1972; 30(2): 481-485.
- Blasco de Villalonga M, Llarena Ibarguren R, Acha Perez M, et al. [Testicular metastasis of renal adenocarcinoma. Report of a case]. Archivos espanoles de urologia. 1994; 47(3): 281-282.
- Daniels GF, Jr., Schaeffer AJ. Renal cell carcinoma involving penis and testis: unusual initial presentations of metastatic disease. Urology. 1991; 37(4): 369-373.
- Datta MW, Ulbright TM, Young RH. Renal cell carcinoma metastatic to the testis and its adnexa: a report of five cases including three that accounted for the initial clinical presentation. International journal of surgical pathology. 2001; 9(1): 49-56.
- Haupt HM, Mann RB, Trump DL, et al. Metastatic carcinoma involving the testis. Clinical and pathologic distinction from primary testicular neoplasms. Cancer. 1984; 54(4): 709-714.
- Johnson DE, Jackson L, Ayala AG. Secondary carcinoma of the testis. Southern medical journal. 1971; 64(9): 1128-1130.
- Moriyama S, Takeshita H, Adachi A, et al. Simultaneous bilateral testicular metastases from renal clear cell carcinoma: A case report and review of the literature. Oncology letters. 2014; 7(4): 1273-1275.
- Nataf R, Rigondet G, Briquel P. [Testicular metastasis from a renal adenocarcinoma. Apropos of 2 recent cases]. Journal d'urologie et de nephrologie. 1975; 81(10-11): 833-843.
- Post GJ, Kassis J. Solitary testicular metastasis from renal cell carcinoma and concomitant primary adenocarcinoma of the prostate. The West Virginia medical journal. 1980; 76(5): 99-102.
- Price EB, Jr., Mostofi FK. Secondary carcinoma of the testis. Cancer. 1957; 10(3): 592-595.
- Rouvinov K, Neulander EZ, Kan E, et al. Testicular Metastasis from Renal Cell Carcinoma: A Case Report and Review of the Literature. Case reports in oncology. 2017; 10(1): 388-391.
- Selli C, Woodard BH, Paulson DF. Late intrascrotal metastases from renal cell carcinoma. Urology. 1982; 20(4): 423-425.
- Hatoum HA, Abi Saad GS, Otrock ZK, et al. Metastasis of colorectal carcinoma to the testes: clinical presentation and possible pathways. International journal of clinical oncology. 2011; 16(3): 203-209.
- Pratap Singh A, Kumar A, Dhar A, et al. Advanced colorectal carcinoma with testicular metastasis in an adolescent: a case report. Journal of medical case reports. 2018; 12(1): 304.
- Verma N, Babu S, Kushwaha JK, et al. Testicular metastasis of colorectal carcinoma: an unusual presentation. BMJ case reports. 2013; 2013.
- Buck DA, Byrd RH, Holmes CL, et al. Testicular metastasis in a case of squamous cell carcinoma of the lung. Case reports in oncology. 2015; 8(1): 133-137.
- Uchida K, Kurimura Y, Miyake M, et al. Testicular metastasis from squamous cell carcinoma of the lung. International journal of urology : official journal of the Japanese Urological Association. 2003; 10(6): 350-352.
- Kaplan MA, Kucukoner M, Inal A, et al. Testicular Mass: An Initial Sign of Squamous Cell Carcinoma of the Lung. World journal of oncology. 2012; 3(6): 291-293.
- Bunn R, Liu S, Stokes S, et al. Mucinous Lung Adenocarcinoma Metastasis to Testis in a 29 Year Old-A Case Report. Urology. 2018; 118: 3-5.
- Doherty AP, Smith R, Paradinas FJ, et al. A case of metastatic transitional cell carcinoma in the testis histologically mimicking intratubular germ cell neoplasia. British journal of urology. 1996; 78(1): 137-138.
- Oppong FC, Rundle JH. Bilateral testicular secondaries from transitional cell carcinoma of the bladder. British journal of urology. 1991; 68(4): 430.
- Watkin NA, Gallegos CR, Moisey CU, et al. Metastatic bladder cancer involving the testis. British journal of urology. 1994; 74(3): 380.
- Kao CS, Ulbright TM, Young RH, et al. Testicular embryonal carcinoma: a morphologic study of 180 cases highlighting unusual and unemphasized aspects. The American journal of surgical pathology. 2014; 38(5): 689-697.
- Ye H, Ulbright TM. Difficult differential diagnoses in testicular pathology. Archives of pathology & laboratory medicine. 2012; 136(4): 435-446.
- Wang F, Liu A, Peng Y, et al. Diagnostic utility of SALL4 in extragonadal yolk sac tumors: an immunohistochemical study of 59 cases with comparison to placental-like alkaline phosphatase, alpha-fetoprotein, and glypican-3. The American journal of surgical pathology. 2009; 33(10): 1529-1539.
- Howitt BE, Magers MJ, Rice KR, et al. Many postchemotherapy sarcomatous tumors in patients with testicular germ cell tumors are sarcomatoid yolk sac tumors: a study of 33 cases. The American journal of surgical pathology. 2015; 39(2): 251-259.
- Magers MJ, Kao CS, Cole CD, et al. "Somatic-type" malignancies arising from testicular germ cell tumors: a clinicopathologic study of 124 cases with emphasis on glandular tumors supporting frequent yolk sac tumor origin. The American journal of surgical pathology. 2014; 38(10): 1396-1409.
- Alexiev BA, Xu LF, Heath JE, et al. Adenomatoid tumor of the testis with intratesticular growth: a case report and review of the literature. International journal of surgical pathology. 2011; 19(6): 838-842.
- Skinnider BF, Young RH. Infarcted adenomatoid tumor: a report of five cases of a facet of a benign neoplasm that may cause diagnostic difficulty. The American journal of surgical pathology. 2004; 28(1): 77-83.
- Williams SB, Han M, Jones R, et al. Adenomatoid tumor of the testes. Urology. 2004; 63(4): 779-781.
- Chekol SS, Sun CC. Malignant mesothelioma of the tunica vaginalis testis: diagnostic studies and differential diagnosis. Archives of pathology & laboratory medicine. 2012; 136(1): 113-117.
- Winstanley AM, Landon G, Berney D, et al. The immunohistochemical profile of malignant mesotheliomas of the tunica vaginalis: a study of 20 cases. The American journal of surgical pathology. 2006; 30(1): 1-6.
- Amin MB. Selected other problematic testicular and paratesticular lesions: rete testis neoplasms and pseudotumors, mesothelial lesions and secondary tumors. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2005; 18 Suppl 2: S131-145.
- Ramsey ML, Yuh BJ, Johnson MT, et al. Carbonic anhydrase IX is expressed in mesothelioma and metastatic clear cell renal cell carcinoma of the lung. Virchows Archiv : an international journal of pathology. 2012; 460(1): 89-93.
- Tong GX, Memeo L, Colarossi C, et al. PAX8 and PAX2 immunostaining facilitates the diagnosis of primary epithelial neoplasms of the male genital tract. The American journal of surgical pathology. 2011; 35(10): 1473-1483.
- Iczkowski KA, Bostwick DG, Roche PC, et al. Inhibin A is a sensitive and specific marker for testicular sex cord-stromal tumors. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1998; 11(8): 774-779.
- McCluggage WG, Shanks JH, Whiteside C, et al. Immunohistochemical study of testicular sex cord-stromal tumors, including staining with anti-inhibin antibody. The American journal of surgical pathology. 1998; 22(5): 615-619.
- Niehans GA, Manivel JC, Copland GT, et al. Immunohistochemistry of germ cell and trophoblastic neoplasms. Cancer. 1988; 62(6): 1113-1123.
- Tickoo SK, Hutchinson B, Bacik J, et al. Testicular seminoma: a clinicopathologic and immunohistochemical study of 105 cases with special reference to seminomas with atypical features. International journal of surgical pathology. 2002; 10(1): 23-32.
