Current Clinical Evidence for Agents used in Acanthamoeba Keratitis: Systematic Literature Reviews
Danielle H. Bodicoat1, Vincenzo Papa2, Rita Alves1, Angela Arteaga Duarte1, Luca Tofani2
1HEOR Value Hub, Brussels, Belgium
2SIFI S.p.A., 36, Via Ercole Patti, 95025 Aci S. Antonio (Catania), Italy
Abstract
To describe the evidence for agents with anti-amoebic activity used to treat Acanthamoeba keratitis (AK), two systematic literature reviews (SLRs) were conducted of 1) clinical outcomes for patients with AK and 2) health economic outcomes for patients with AK or microbial keratitis (MK).
The intervention of interest was any agent with anti-amoebic activity administered as eye-drops or orally. The main outcome was clinical resolution. Electronic databases (January 1992-July 2022), conference abstracts (2017-2022), and relevant websites were hand-searched. Risk of bias assessments used external assessment tools. A narrative synthesis was conducted.
The clinical SLR (37 studies; 2043 patients) identified at least 20 studies reporting clinical resolution, best-corrected visual acuity, and corneal surgery; fewer studies reported other outcomes. Treatment regimes, outcome definitions and assessment timing varied markedly between studies. Studies classified as fair or poor quality appeared to underestimate the burden of AK compared with good quality studies. For health economic outcomes (15 studies; 1878 patients), very limited evidence in AK populations was found.
In conclusion, there was a substantial amount of clinical evidence, but scarce economic evidence. Study quality and comparability challenges should be considered when estimating the impact of AK, with substantial between-study heterogeneity limiting options for robust evidence synthesis.
Introduction
Acanthamoeba keratitis (AK) is a rare, but potentially devastating, microbial keratitis (MK) caused by Acanthamoeba spp, an ubiquitous free-living amoeba1. AK typically presents sub-acutely or chronically, and approximately 90% of cases occur in contact lens wearers2-5. Patients with AK may suffer from pain, which may be disproportionate to clinical findings1. AK is associated with photophobia, blurred vision, and tearing1. Successful management of AK depends on early diagnosis and prompt aggressive therapy. The natural history of untreated AK is characterized by poor vision, potential blindness, and the need for surgical procedures, such as keratoplasty or enucleation.
There are currently no approved medicinal products or clinical guidelines for treating AK6. Current management approaches typically use combination therapy of biguanides (polihexanide or chlorhexidine) and diamidines (propamidine or hexamidine)1-4,6. Polihexanide (PHMB) and chlorhexidine are generally administered at a concentration of 0.02% (or 0.2 mg/ml). Diamidines are administered at a concentration of 0.1% (or 1 mg/ml). Corticosteroids may also be used, even though their use in AK is controversial1,2,4. Therapeutic keratoplasty is used in the severe form of AK when topical treatment has failed1,2.
To our knowledge, only a single published systematic literature review (SLR) on agents with anti-amoebic activity for AK exists, which was published over eight years ago so may not be up-to-date7. This previous SLR also only included randomized controlled trials (RCTs), of which only one eligible RCT comparing two treatments was identified7. Additionally, a scoping review has recently been published, which identified that agents with anti-amoebic activity are typically only prescribed for patients with AK after other treatments have been exhausted6. No evidence syntheses using meta-analysis or indirect treatment comparisons were identified.
To describe the evidence base available for current, unlicensed agents with anti-amoebic activity used to treat AK, two SLRs were conducted to identify studies reporting 1) clinical outcomes for patients with AK (the primary focus of this paper), and 2) health economic outcomes for patients with AK or MK (the secondary focus of this paper). This will help to understand the availability of evidence for agents with anti-amoebic activity in patients with AK, key findings across these studies, and whether future evidence syntheses can be conducted.
Methods
The protocols were guided by an initial targeted literature review (TLR), scoping reviews, and PRISMA-P guidance8. The protocols were signed off and registered on PROSPERO (CRD42022345288, CRD42022345290) before the searches were conducted. During protocol development, it was found that very few studies reported health economic outcomes in AK so the population for health economic outcomes was widened to also cover MK and two separate, but very similar, SLRs were conducted. The methodology for the clinical SLR is described below. The methodology for the economic SLR is provided in Supplementary Material 1.
Eligibility criteria
Full eligibility criteria are described in Supplementary Material 2. In brief, the population of interest was patients of any age with a confirmed diagnosis of AK. The intervention of interest was any agent with anti-amoebic activity administered as eye-drops or orally in any concentration or combination. The control of interest was any control (including other active eye drop medication or placebo) or no control (i.e., single-arm study). Eligible studies were those that reported at least one efficacy or safety outcome of interest (see Supplementary Material 2 for the full list). Clinical resolution (cure) was the primary outcome of interest, with time-to-cure and best-corrected visual acuity (BCVA) as the main secondary outcomes of interest. Any clinical trial or observational study with ≥5 participants and published from January 1992 onwards was potentially eligible.
Literature search methodology
Searches were implemented in electronic databases (PubMed; Cochrane Database of Systematic Reviews; Prospero International Prospective Register of Systematic Reviews; Cochrane Central Register of Controlled Trials; ClinicalTrials.gov). Additionally, conference proceedings from 2017-2022 (Association for Research in Vision and Ophthalmology, European Association for Vision and Eye Research, American Association of Ophthalmology, American Society of Corneal and Refractive Surgery, European Society of Corneal and Refractive Surgeons, Cornea Society) and the Summary of Product Characteristics and websites of relevant medications were hand-searched. The list of eligible articles from the TLR was obtained.
Potentially eligible studies from all sources were combined into a de-duplicated list. Titles and abstracts were independently reviewed against the eligibility criteria by two reviewers (DHB, AAD), who discussed any conflicts. Where agreement on inclusion was not reached, the record proceeded to the next stage. Full text articles were retrieved for publications that remained potentially eligible. The same two reviewers then independently reviewed the full texts against the eligibility criteria to identify eligible publications. The reviewers discussed any conflicts and decided whether the study was eligible or not. Where an agreement could not be made, a third reviewer (AAD) held the casting vote.
Where the search identified an SLR with a population with AK, a single reviewer (DHB) hand-searched its reference list for potentially eligible articles. Additionally, for eligible full-text articles, a single reviewer (DHB) hand-searched the reference list and conducted forward citation searching in Google Scholar to identify further potentially eligible articles. These additional articles went through the same process of title/abstract screening followed by full-text screening by two independent reviewers. If any of these articles were eligible, subsequent reference list hand-searching and forward citation searching was conducted. This circular process was repeated until no new relevant articles were identified.
Microsoft Excel and Covidence were used to track the literature search.
Search strategies
Searches were conducted on 25th and 26th July 2022. The PubMed and Cochrane Library search strategies are provided in Supplementary Material 2. Scoping reviews in the other electronic databases found that fewer than ten records were returned for free text and MeSH terms for AK so all records pertaining to AK from these databases were included in the title/abstract screening.
Data extraction
A Microsoft Excel data extraction form was created that included the outcomes of interest (see Supplementary Material 2) and other relevant datapoints on the publication (e.g., first author, publication year), study (e.g., country, study design), participants (e.g., population description, demographics), and interventions (e.g., drug name and concentration, total duration of use). This form was piloted with three randomly selected articles and refined. If substantial refinement was required, the data extraction form was re-piloted on another three randomly selected articles. Three rounds of piloting were conducted for each data extraction form.
One reviewer (DHB) undertook data extraction based on the published information available, with a second reviewer (AAD) checking the extracted data against the original publications. The reviewers discussed and agreed on any conflicts.
For all outcomes, the timepoint of assessment and outcome definition were extracted. Where outcomes were reported at multiple timepoints, every timepoint was extracted. Where it was not stated whether data were reported at patient-level or eye-level, it was assumed that data were reported at eye-level, unless there was evidence to contradict this assumption in the manuscript. Data not available in the published report were marked as missing, except for clinical resolution for which the corresponding author of each eligible study was emailed asking for 1) any clinical resolution data where these were not in the published article, or 2) clinical resolution data that matched the definition used in the polihexanide 0.08% pivotal trial9 where a different definition was used in the published article. The latter request was to facilitate a planned future evidence synthesis.
Risk of bias assessment
Study-level risk of bias was independently assessed using the National Heart, Lung, and Blood Institute’s Study Quality Assessment Tools10 by two reviewers (DHB, AAD). After completing the assessment, the reviewers discussed any conflicts reaching an agreement in all cases of initial conflict. The reviewers agreed an overall rating for risk of bias (good, fair, poor) for each study based on the study’s individual scores. Following the Study Quality Assessment Tools guidance, the overall rating was based on a subjective assessment of the risk of bias10.
Synthesis methods
A narrative synthesis was conducted where the amount, type, and quality of data, along with key findings, were summarized at eye-level. The narrative synthesis focused on the main outcomes of interest (clinical resolution, time-to-cure, BCVA), with a shorter summary for other clinical outcomes.
To summarize outcomes that were sufficiently comparable across studies, the range (weighted mean) of values at eye-level across treatment arms with ≥5 treated eyes are reported. For the main outcomes, the weighted mean was also reported in subgroups depending on the overall study quality (good vs fair/poor).
Where individual patient data were reported for BCVA, the following outcomes were calculated where possible: 1) Improvement in BCVA from baseline to measured timepoint (yes/no), and 2) Number of patients with BCVA of 6/12 or better. Where data were reported in weeks, these were converted into days as weeks multiplied by 7. Where data were reported in months, these were converted into days as months multiplied by 28.
Certainty of evidence
The strength of the overall body of evidence for each outcome was assessed by one reviewer (DHB) using the GRADE framework11. GRADE ratings are: ‘very low’ (true effect is probably markedly different from the estimated effect); ‘low’ (true effect might be markedly different from the estimated effect); ‘moderate’ (true effect is probably close to the estimated effect); and ‘high’ (authors have a lot of confidence that the true effect is similar to the estimated effect)11.
Changes from protocol
When a manufacturer’s website could not be identified for a treatment of interest, another reputable website with relevant product information was hand-searched instead. A search of the website for Impavido (US label for oral miltefosine) was added to the search strategy. When a non-English language article had an English language abstract available, screening and data extraction were performed on the abstract alone.
Results
There follows a description of the clinical SLR results. The economic SLR results are provided in Supplementary Material 1.
Description of studies
After full text screening, 37 articles were eligible (Figure 1). The initial agreement between the reviewers for the title and abstract screening was 88.2% (Cohen’s kappa = 0.75) and for the full text screening was 85.5% (Cohen’s kappa = 0.71). The eligible articles were published between 1993-2022, and their characteristics are shown in Supplementary Material 3.
There were two RCTs, 24 retrospective analyses of routine data, seven prospective single-arm interventional studies, two case-control studies, and two cohort studies. Average follow-up length was 85 to 4782 days. Most studies were conducted in Europe (n=18) or Asia (n=12).
The studies included 2043 patients (mean age: 21.1-53.0 years). The percentage of males was 0 to 82% (weighted mean: 43.0%). Only one study reported ethnicity12. In 30 studies, the inclusion criteria covered any patient with AK, whereas the remaining studies focused on subgroups of AK patients.
Eleven studies reported disease stage at baseline, with most studies including a mix of disease stages. Disease stage definitions differed somewhat between studies. Mean time from symptom initiation to diagnosis ranged between 7.1-81.6 days. The percentage of contact lens wearers ranged between 0-100% (weighted mean: 64.9%), of bilateral keratitis between 0-20% (weighted mean: 3.3%), and of patients who used corticosteroids before the study treatment between 0-69% (weighted mean: 21.8%).
Treatment regimens were greatly heterogeneous across the studies, with at least 31 different regimens used. Polihexanide (typically 0.02%) was given as a monotherapy in eight studies, and in combination with other treatments (mostly propamidine 0.1%) in 22 studies. Chlorhexidine was given as a monotherapy in seven studies, and in combination with other treatments (mostly propamidine 0.1%) in 16 studies. Five (13.2%) studies had no arms that included a biguanide.
There were 21 studies rated as ‘good’ for overall quality, 10 as ‘fair’, and six as ‘poor’ (data not shown).
Clinical resolution
Clinical resolution was reported in 20 studies (34 treatment arms; 634 patients; 643 eyes; GRADE score = moderate; Table 1). Studies reported clinical resolution at 12 months (n=2), four months (n=1), three months (n=1), after completion of medical therapy (n=3), at ‘final follow-up’ without specifying the timing of final follow-up (n=9), or an unreported timepoint (n=4).
Clinical resolution definitions differed between studies, with no clear definition provided in three studies. Most definitions included or implied a healed cornea without inflammation and after discontinuation of an agent with anti-amoebic activity, regardless of the level of vision.
In 22 treatment arms with ≥5 treated eyes, the percentage cured (based on the study’s own definition) was 4.0% to 100.0% (weighted mean: 60.1%) and <50% of patients were cured in six treatment arms (27.3%). In subgroup analyses by study quality, the weighted mean was 57.6% cured in 12 good quality treatment arms compared with 66.0% in the 10 fair or poor quality arms.
Time-to-cure
Time-to-cure was reported in five studies (97 patients; 101 eyes; GRADE score = low; Table 2). Because the definition of cure differed between studies (Table 1), the time-to-cure endpoint may not be directly comparable between studies.
In treatment arms with ≥5 treated eyes, the mean time-to-cure ranged from 73 to 309 days (weighted mean: 140.7 days; four study arms), and median ranged from 52 to 196 days (weighted mean: 150.7 days; two study arms). The weighted mean of the mean time-to-cure was 154.2 days in good quality studies and 105.8 in lower quality studies. Only one good and lower quality study each reported median time-to-cure, so subgroup analyses by study quality were not performed for median time-to-cure.
Best-corrected visual acuity (BCVA)
BCVA reporting varied across the studies, with continuous and categorical outcomes reported (Supplementary Material 4). Overall, the GRADE quality of evidence for BCVA was moderate.
LogMAR BCVA was reported in eight studies (208 patients; 215 eyes) at: final follow-up (n=4); after treatment (n=2); multiple timepoints (n=1); and unreported timepoint (n=1). BCVA was reported as a change from baseline (n=1) or absolute value (n=7). Most studies (7 out of 8) reported absolute values at one or more follow-up points, and 6 out of 7 of these reported mean values (the remaining study reported median values), so the mean absolute follow-up (the timings of which differed as described above) BCVA values were summarized. In treatment arms with ≥5 treated eyes (n=8), the mean absolute follow-up BCVA ranged from 0.03 to 0.89 (weighted mean: 0.5). These studies were all rated as good quality.
BCVA as a categorical variable was reported in 24 studies (908 patients) at: final follow-up (n=15); unreported timepoint (n=4); end of treatment (n=2); multiple timepoints (n=1); two weeks (n=1); and four months (n=1). There were different definitions, with the most commonly available being improvement in BCVA from baseline (n=10 studies) and BCVA of 6/12 or better on a Snellen scale (n=13 studies).
In 11 treatment arms with ≥5 treated eyes, the percentage of patients with improved BCVA ranged from 38.5% to 100.0% (weighted mean: 72.9%). In subgroup analyses, the weighted mean was 65.1% for the seven good quality treatment arms, and 87.6% for the four treatment arms from fair or poor quality studies.
The percentage of patients with a follow-up BCVA of 6/12 or better, in the treatment arms with ≥5 treated eyes (n=15), ranged from 29.0% to 100.0% (weighted mean: 78.7%). For good quality studies, the weighted mean was 76.8% (6 treatment arms), with a higher weighted mean of 80.0% for fair and poor quality studies (9 treatment arms).
Other clinical outcomes
Table 3 shows the other reported clinical outcomes. Again, a consistent theme across the outcomes was that the outcome definitions and timing of measurement differed greatly.
Economic SLR results
Supplementary Material 1 provides the full results of the economic SLR. In summary, after full text screening, 15 articles (published 1993-2022) were eligible, of which seven included a population with AK and the other eight included other populations with MK. Again, treatment regimens varied greatly across the studies. Of the 15 eligible studies, 9 were rated as ‘good’ for overall quality, five as ‘fair’, and one as ‘poor’.
Four observational studies reported direct costs data. None of these studies were in a population with AK. Costs were not reported by treatment in three of the studies, and each study emanated from a different country, hindering comparability. The reported outcomes, outcome measures, currencies, and populations differed substantially between studies. These differences all likely contributed to the strong divergence on estimates for direct costs across studies (see Supplementary Material 1).
Resource use was the most reported economic outcome. Seven observational studies reported length of hospital stay for 543 patients, three of which were in patients with AK. Length of hospital stay ranged from 0 to 80 days, with the mean values ranging from 0 to 15 days. The study population appeared to have some impact on length of hospital stay, with two of the three AK studies having the highest mean length of stay across all seven studies and the highest number of days in hospital (80 days) was in a patient with AK.
Additionally, eight observational studies (1238 patients; four studies in patients with AK) reported total (range = 2 – 70) and/or mean (range = 2 - 29) number of outpatient hospital visits, and five observational studies (584 patients; three studies in patients with AK) reported the percentage of patients admitted to hospital (range = 13% - 93%).
The GRADE quality of evidence for length of hospital stay and outpatient hospital visits was low, and for direct costs and hospital admissions was very low.
Discussion
Two SLRs were conducted to understand the existing evidence for agents with anti-amoebic activity used in AK, one focusing on clinical evidence and the other on economic evidence.
Amount and quality of data
In the clinical SLR, there were 37 eligible studies (2043 patients). The number of studies reporting the main outcome of interest (clinical resolution) was fairly high (n=20 studies), and there was also a large body of evidence for BCVA (n=28 studies) and corneal surgery (n=25 studies). There was a low to moderate number of studies for the other reported outcomes. Based on the GRADE assessment, the overall certainty of evidence was moderate for clinical resolution, BCVA, corneal surgery, and corneal scarring. However, the certainty for the other outcomes was rated as low to very low. None of the clinical outcomes had a GRADE rating of ‘high’ for the overall certainty of evidence. Most studies (35 out of 37) were observational studies. This aligns with the findings of a previous SLR in this indication, which only included RCTs and identified only one eligible study when it was published eight years ago7. Most studies were rated as ‘good’ or ‘fair’ for overall quality5,9,12-39, with only six studies rated as ‘poor’40-45. When considering the rarity of this disease, this could represent a substantial amount of reasonably good clinical evidence overall.
There was much less economic data available, particularly in populations with AK. Despite widening the population of interest to include all patients with MK and conducting thorough searching, outcomes were only reported in a small number of studies (n=1 to 8). The number of outpatient visits was the most commonly reported outcome. There were several outcomes of interest with no data available. Any data from populations with MK, rather than AK, may not be directly transferable to those with AK. However, such data may be able to provide indicative estimates of economic burden in areas where there are no such data for AK. Finally, the evidence for the economic outcomes was scored as having ‘low’ or ‘very low’ certainty of evidence using the GRADE framework11, predominantly because the evidence only came from observational studies. Most of the studies were rated as ‘good’ or ‘fair’ for overall quality, with only one study (a prospective single-arm interventional studies) rated as ‘poor’46, suggesting that the studies themselves were of reasonable quality. The overall lack of data, particularly in patients with AK, severely limits our understanding of the economic impact of AK.
Clinical findings
Across all treatment arms with ≥5 treated eyes, the weighted mean of the percentage cured was 60.1% (range: 4% to 100%). This accords with a recent scoping review which also found that the AK continues to be associated with unfavourable outcomes6. Subgroup analyses suggested that the percentage was lower in good quality (57.6%) than fair or poor quality (66.0%) studies. Similarly, the weighted mean of the mean time-to-cure was higher in good quality (154.2 days) then fair or poor quality studies (105.8 days), of the percentage with improved BCVA was lower in good quality (65.1%) than fair or poor quality (87.6%) studies, and of the percentage with a follow-up BCVA of 6/12 or better was lower in good quality (76.8%) than fair or poor quality studies (80.0%). Whilst the high level of heterogeneity between studies means that these results should be interpreted with caution, these findings suggest that fair or poor quality studies may underestimate the clinical impact of AK on patients and that study quality should be considered when interpreting the results of studies in patients with AK as the findings may be misleading.
There were no studies that stated that no enucleations occurred, which could imply that enucleation data were only reported in studies where at least one enucleation occurred. If that were the case, and the studies that did not report this outcome had zero enucleations, then using the data reported in the literature to estimate the rate of enucleation would give an overestimate.
Comparability of outcomes
The comparability of clinical resolution outcomes were of particular interest given that this is a key outcome for patients and treating clinicians. Twenty studies reported clinical resolution (691 patients)12,13,15,18,19,23,24,28-30,32,33,35-38,40,41,45. The definition of clinical resolution and timing of measurement differed across the studies making comparison between them challenging. An important difference between definitions was the handling of discontinuations. Some studies considered discontinuation of the initial treatment for any reason as treatment ‘failure’ so patients who discontinued the initial treatment were not counted as cured. Other studies did not clearly state whether patients who discontinued the initial treatment were considered as ‘cured’ or ‘failed’. Studies where treatment discontinuations were not considered as failures may have an artificially higher cure rate than those that do consider treatment discontinuations as failures.
Whilst there was a substantial amount of data relating to BCVA, BCVA was reported in very varied ways across the eligible studies. Eight studies reported LogMAR BCVA13-15,17,19,29,33,47 and 24 studies reported BCVA as a categorical variable on a LogMAR or Snellen scale5,14,18-21,25-33,35-37,39,41-44,48. A crucial difference in BCVA definition across the studies was that the timing of measurement was not always clear, particularly because many studies reported ‘final BCVA’ without reporting when this final measurement occurred. An important consequence of this is that it was also not always clear whether optical surgery occurred before BCVA was measured, or how long the patient received medical treatment for prior to the BCVA measurement being recorded.
There were substantial differences in outcome definitions, timing of measurements, and treatment regiments for most clinical and economic outcomes meaning that there are major limitations in how this evidence can be compared and synthesized. Conversely, the study populations were broadly similar for the clinical outcome studies, with similar definitions used for AK diagnosis across the studies. This suggests that more standard outcome definitions should be created for studies investigating agents with anti-amoebic activity used in AK to allow better comparison of treatments in the future and to better understand the burden of AK.
Limitations
The main limitation of the clinical review was that the list of outcomes was wide and varied. This was intentional to capture all relevant evidence to understand the depth and context of the available evidence. However, this resulted in an evidence-base that is somewhat divergent in terms of the reported outcomes, their definitions, and assessment timings. This means that the summary statistics in this paper are better interpreted as indicators of patterns because the results are based on a naïve synthesis approach, different outcome definitions and timings, and patients could have switched treatments during the study.
For the economic review, the population of interest had to be widened during protocol development beyond AK patients (the primary condition of interest) to also include MK patients, due to a lack of relevant evidence in AK. This adds uncertainty due to the potential lack of transferability of data from a MK population to an AK population, so the findings in different populations should be seen as indicative, rather than definitive.
The proceedings from 2017-2022 of six leading conferences were hand-searched for potentially eligible abstracts. The list of conferences was based on clinical advice on the leading conferences for AK. This list may not be exhaustive. The impact of any missing key conferences on the completeness of the SLR is likely to be low because other eligible conference abstracts should have been identified through the iterative backward and forward citation searching.
Conclusions
Two SLRs were conducted to understand existing clinical and health economic evidence for the unlicensed agents with anti-amoebic activity currently used for AK. There was a fairly substantial body of evidence for clinical outcomes, with most studies rated as ‘good’ or ‘fair’ for overall quality. However, substantial differences in treatment regimens, outcome definitions, and timing of measurements mean that there are major limitations in how this evidence can be compared and synthesized making it difficult to determine the effectiveness of treatments in AK. There was a suggestion that fair or poor quality studies may underestimate the burden of AK compared with good quality studies leading to biased results.
For health economic outcomes, there is very limited evidence in AK populations, which all comes from observational studies, is focused on resource use rather than direct costs, and reports outcomes inconsistently between studies. This limits overall understanding of the cost-effectiveness of agents with anti-amoebic activity that are used to treat AK, which should be a focus of future research. There is some additional evidence from populations with MK, however this may not be transferable to populations with AK.
No other published evidence syntheses on this topic were identified during the search. Such estimates are needed to understand how best to treat AK, but any future syntheses will be limited by, and need to account for, the inconsistencies in outcomes, treatment regimens, and populations across the studies. A feasibility assessment for an indirect treatment comparison is planned to determine whether an evidence synthesis can be conducted using the existing evidence base for agents with anti-amoebic activity in people with AK.
List of abbreviations: AK, Acanthamoeba keratitis; BCVA, Best-Corrected Visual Acuity; MK, Microbial keratitis; RCT, Randomized Controlled Trial; SLR, Systematic Literature Review; TLR, Targeted Literature Review.
Competing Interests: SIFI S.p.A. manufacture polihexanide 0.08%.
Funding: The work was funded by SIFI S.p.A., 36, Via Ercole Patti, 95025 Aci S. Antonio (Catania), Italy.
Author Contributions: All authors contributed to the design of the review and writing of the manuscript. DHB, RA and AAD conducted the searches, screening and data extraction.
Table 1: Crude cure rates (without adjustment for any baseline risk factors) for medical cures (without surgery) reported in eligible studies with outcomes for a defined regime including at least one agent with anti-amoebic activitya and without adjusting for adjunctive anti-inflammatory therapy.
|
Study (Quality rating) |
Treatment |
Definition (Timepoint) |
N patient (Eyes) |
N (%) cured |
|
Randomized controlled trial |
||||
|
Bagga, 202113,b (Fair) |
PHMB 0.02% & CHX 0.02% |
Completely resolved (Final follow-up) |
10 (10) |
4 (40.0) |
|
VRC 1% |
8 (8) |
4 (50.0) |
||
|
Retrospective analysis of routine data |
||||
|
Bonini, 202115 (Good) |
PHMB 0.02% & PD 0.1% |
Complete corneal healing (Final follow-up) |
27 (31) |
23 (74.2) |
|
Megha, 202018 (Good) |
PHMB 0.02% |
Complete healing with corneal vascularization and scarring (Final follow-up) |
11 (10) |
7 (70.0) |
|
Musayeva, 202019 (Good) |
PHMB 0.02%, PD 0.1%, VRC 1% & others |
No signs of infection (After medical therapy) |
26 (26) |
26 (100.0) |
|
Papa, 202012,c (Good) |
PHMB 0.02% & diamidine 0.1% |
Cure without surgery, independent of visual acuity, defined as clinical evidence of elimination of Acanthamoeba- an intact corneal epithelium with no clinical signs of ocular inflammation after discontinuing anti-amoebic agent for 30 days, and treating discontinuations of baseline anti-amoebic agent as failures (12 months) |
114 (114) |
61 (53.5) |
|
Diamidine 0.1% |
25 (25) |
1 (4.0) |
||
|
PHMB 0.02% |
50 (50) |
22 (44.0) |
||
|
Various |
38 (38) |
15 (39.5) |
||
|
Zhong, 201723 (Good) |
Neomycin 0.5% & PD 0.1% |
Corneal epithelium recovered and ulceration resolved with scarring (After medical therapy) |
15 (15) |
1 (6.7) |
|
Jiang, 201524 (Good) |
PHMB 0.04% & CHX 0.04% |
Cured with corneal scarring (After medical therapy) |
70 (70) |
43 (61.4) |
|
Mathers, 200640 (Poor) |
CHX 0.02% or 0.04% then 0.06% |
NR (NR) |
8 (8) |
7 (87.5) |
|
Sun, 200628,d (Good) |
CHX 0.02%, neomycin 0.5% & metronidazole 0.4% |
Corneal infiltrates and ulcerations resolved with haze or scarring (Final follow-up) |
3 (3) |
2 (66.7) |
|
CHX 0.02%, neomycin 0.5%, metronidazole 0.4% & CHX 0.1% 2x weekly |
17 (17) |
17 (100.0) |
||
|
Perez- Santonja, 200329 (Good) |
PHMB 0.02% & hexamidine 0.1% |
Disease resolved with residual corneal scarring and vascularisation (Final follow-up) |
2 (2) |
1 (50.0) |
|
PHMB 0.02%, hexamidine 0.1% & CHX 0.02% |
4 (4) |
1 (25.0) |
||
|
PHMB 0.02% |
1 (1) |
0 (0.0) |
||
|
PHMB 0.02% & PD 0.1% |
1 (1) |
1 (100.0) |
||
|
Donoso, 200241 (Poor) |
PHMB 0.02% & PDe |
Infection irradicated (Final follow-up) |
27 (31) |
31 (100.0) |
|
Azuara- Blanco, 199730 (Good) |
PHMB 0.02%, PD 0.1% & polymyxin plus bacitracine |
Eye had stable corneal signs and no inflammation for at least 3 consecutive months after cessation of all treatment (Final follow-up) |
9 (9) |
9 (100.0) |
|
CHX 0.02% (then PHMB 0.02%), PD 0.1% & polymyxin plus bacitracine |
1 (1) |
1 (100.0) |
||
|
Skarin, 199631 (Fair) |
PD 0.1%, neomycin / polymyxin Be & oral ketoconazole 200 mg |
Healed without surgery (12 months) |
4 (4) |
3 (75.0) |
|
PD 0.1% & neomycin / polymyxin Be |
1 (1) |
1 (100.0) |
||
|
PD 0.1%, neomycin/polymyxin Be, oral ketoconazole 200 mg, miconazol 2% & paromomycin 1.5% or 2.5% |
1 (1) |
1 (100.0) |
||
|
PD 0.1%, neomycin/polymyxin Be, oral ketoconazole 200 mg, paromomycin 1.5% or 2.5% & PHMBe |
1 (1) |
1 (100.0) |
||
|
PD 0.1%, neomycin/polymyxin Be, oral ketoconazole 200 mg, paromomycin 1.5% or 2.5%, PHMBe & clotrimazol 1.5% |
1 (1) |
0 (0.0) |
||
|
Elder, 199432 (Fair) |
Neomycin 0.5% & PD 0.1% |
No inflammation and stable corneal signs for 6 consecutive months or more after all treatment ceased (NR) |
19 (19) |
9 (47.4) |
|
PHMB 0.02% & PD 0.1% |
4 (4) |
4 (100.0) |
||
|
Prospective single-arm interventional study |
||||
|
Caruso, 202033 (Good) |
CHX 0.02% & Vitamin E TGPS 0.2% |
No corneal inflammation (3 months) |
29 (29) |
25 (86.2) |
|
Revathi, 201845 (Poor) |
PHMB 0.04% & CHX 0.04% |
Healed (NR) |
25 (25) |
15 (60.0) |
|
Hargrave, 199938 (Fair) |
PD 0.1% & NPG |
Cure with adherence to protocol i.e. no surgery or third drug (Final follow-up) |
60 (60) |
30 (50.0) |
|
Kosrirukvongs, 199935 (Fair) |
CHX 0.006% |
Medically cured (NR) |
5 (6) |
5 (83.3) |
|
Seal, 199636,f (Fair) |
CHX 0.02% & PD 0.1% |
Resolution of signs (Final follow-up) |
12 (12) |
11 (91.7) |
|
Varga, 199337 (Fair) |
PHMB 0.02%, PDe & neomycine |
Asymptomatic (4 months) |
5 (6) |
6 (100.0) |
Abbreviations: CHX, Chlorhexidine; NPG, Neomycin – Polymyxin B – Gramicidin; NR, Not reported; PD, Propamidine; PHMB, Polihexanide; VRC, Voriconazole.
a Agent with anti-amoebic activity was given topically unless otherwise stated.
b The number of patients with ‘resolving keratitis’ at the last visit was also reported as 3/10 (30.0%) in the polihexanide and chlorhexidine arm and 3/8 (37.5%) in the VRC arm.
c Data provided by corresponding author in line with the systematic literature review protocol and so these data do not correspond with those in the published article.
d One patient with coinfection of Acanthamoeba and fungus was treated with topical natamycin (5%), which may have an anti-amoebic effect.
e Dose not reported.
f One eye was treated with a therapeutic keratoplasty for secondary bacterial keratitis which was included as a cure in the study because there was no recrudescence of AK in the transplant. It was not included as a clinically cured case due to the therapeutic keratoplasty.
Table 2: Time-to-cure (days) as reported in eligible studies.
|
Study (Quality rating) |
Treatment |
Definition |
N patients (Eyes) |
Mean (SD) days |
Median days |
|
Randomized controlled trial |
|||||
|
Bagga, 202113 (Fair) |
PHMB 0.02% & CHX 0.02% |
Duration taken for clinical resolution |
4 (4) |
NR |
77.5 |
|
VRC 1% |
Duration taken for clinical resolution |
4 (4) |
NR |
52 |
|
|
Retrospective analysis of routine data |
|||||
|
Bonini, 202115,a (Good) |
PHMB 0.02% & PD 0.1% |
Time from clinical diagnosis to complete corneal healing |
19 (23) |
309.1 (255.6) |
196 |
|
Nasef, 202116 (Good) |
PHMB 0.02% & PD 0.1% |
Complete epithelial healing and resolution of the inflammatory signs |
NR (NR) |
73.3 (23.7) |
NR |
|
Prospective single-arm interventional studies |
|||||
|
Revathi, 201845,b (Poor) |
PHMB 0.04% & CHX 0.04% |
Mean healing time |
15 (15) |
126.0 (NR) |
NR |
|
Seal, 199636,b (Fair) |
CHX 0.02% & PD 0.1% |
Signs resolved or stabilised |
11 (11) |
78.3 (47.7) |
56 |
Abbreviations: CHX, Chlorhexidine; NR, Not Reported; PD, Propamidine; PHMB, Polihexanide; SD, Standard Deviation; VRC, Voriconazole.
a Units were reported in months in the original publication and have been converted to days as months * 4 * 7.
b Units were reported in weeks in the original publication and have been converted to days as weeks * 7.
Table 3: Summary of other clinical outcomes as reported in the eligible studies.
|
Outcome, references (GRADE scorea) |
N studies (patients) |
Notes |
Key findings in treatment arms with ≥5 treated eyes |
|
Corneal surgeryb 5,13-16,18-21,23,25-30,32-34,36,38,41,44,45,48 (Moderate) |
25 (874) |
23 studies reported surgery as measured at the final follow-up; 2 studies reported on surgery after medical therapy. Different types of surgery were reported: most reported keratoplasty for therapeutic and/or other reasons (n=19 studies) or “any corneal surgery” (n=4 studies). Some studies stated that patients had keratoplasty without explicitly giving the reason. |
Across 27 arms, the % of patients who had surgery (based on studies that reported “keratoplasty for therapeutic and/or other reasons” or “any corneal surgery”) ranged from 0.0% to 86.7% (weighted mean: 23.7%). |
|
Corneal scarring 14,29,33,37,42 (Moderate) |
5 (105) |
Studies reported corneal scarring at multiple timepoints (n=1), 4 months (n=1), after medical therapy (n=1), across the whole study (n=1), or at an unreported timepoint (n=1). |
Across 5 arms, the % with corneal scarring ranged from 27.3% to 92.9% (weighted mean: 74.2%). |
|
AEs 5,14,17,19,20,28,35-38 (Low) |
9 (292) |
Different types of outcomes were reported, including: serious toxic side effects, corneal toxicity, stinging/burning sensation of the eye, vascularization, secondary glaucoma, and drug toxicity. |
The results cannot be usefully summarised because no two studies used the same AE definition for reporting outcomes. |
|
Adjunctive therapy 16,20,25,29,32,33 (Low) |
6 (95) |
The definition of adjunctive therapy was slightly different in each study, but generally the definitions included the addition of one or more new therapies. |
Across 5 arms, the % of patients who used adjunctive therapy ranged from 3.8% to 52.6% (weighted mean: 18.6%). |
|
Worsening of disease 13,18,34 (Low) |
3 (33) |
There were differing definitions of worsening disease across the studies. |
Across 4 arms, the % with worsening disease ranged from 12.5% to 100.0% (weighted mean: 35.5%). |
|
Enucleation 5,21,29,31,34,35,38,40 (Very low) |
8 (470) |
One study reported enucleation after initial surgery, whilst the other seven studies reported data on enucleation over the total study period. |
There were 14 enucleations in total. Across 7 arms reporting data over the total study period, the % of patients who had enucleation was 0.0% to 20.0% (weighted mean: 2.7%). |
|
Composite endpoints 14,21,22 (Very low) |
3 (453) |
One RCT reported treatment failure at two weeks. The other two studies were retrospective analyses of routine data that reported the composite outcome across the entire study period. |
The definitions of the composite endpoints differed too greatly to allow comparison between studies. |
|
Relapse 5,19,30 (Very low) |
3 (140) |
Each study had a different definition of relapse. |
Across 4 treatment arms, the % that relapsed was 0.0% to 18.1% (weighted mean: 14.3%). |
|
Treatment compliance 5 (Very low) |
1 (19) |
This study reported poor compliance only among patients who relapsed. |
10.5% of patients who relapsed had poor compliance. |
|
VFQ-25 15 (Very low) |
1 (27) |
This study reported the composite VFQ-25 score at the final follow-up. |
Mean VFQ-25 composite score was 80.6 for patients treated with PHMB and propamidine. |
Abbreviations: AE, Adverse Event; PHMB, Polihexanide; RCT, Randomized Controlled Trial.
a GRADE quality of evidence score.
b Other than enucleation.
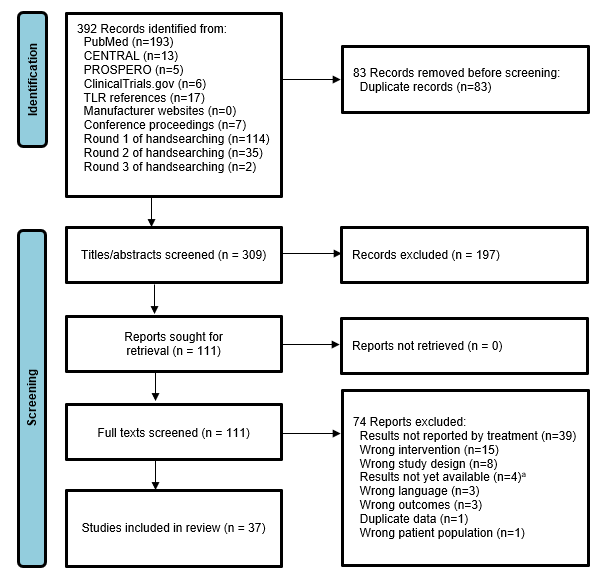
Figure 1: PRISMA diagram showing the flow of studies for the clinical systematic literature review. Abbreviations: TLR, Targeted Literature Review.
References
- Kaufman AR, Tu EY. Advances in the management of Acanthamoeba keratitis: A review of the literature and synthesized algorithmic approach. Ocul Surf. Jul 2022; 25: 26-36. doi: 10.1016/j.jtos.2022.04.003.
- Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015; 22: 10. doi: 10.1051/parasite/2015010.
- Radford CF, Minassian DC, Dart JK. Acanthamoeba keratitis in England and Wales: incidence, outcome, and risk factors. Br J Ophthalmol. May 2002; 86(5): 536-42. doi: 10.1136/bjo.86.5.536.
- Szentmáry N, Daas L, Shi L, et al. Acanthamoeba keratitis – Clinical signs, differential diagnosis and treatment. Journal of Current Ophthalmology. 2019; 31(1): 16-23.
- Duguid IG, Dart JK, Morlet N, et al. Outcome of acanthamoeba keratitis treated with polyhexamethyl biguanide and propamidine. Ophthalmology. Oct 1997; 104(10): 1587-92. doi: 10.1016/s0161-6420(97)30092-x.
- Büchele MLC, Nunes BF, Filippin-Monteiro FB, et al. Diagnosis and treatment of Acanthamoeba Keratitis: A scoping review demonstrating unfavorable outcomes. Cont Lens Anterior Eye. Apr 26 2023: 101844. doi: 10.1016/j.clae.2023.101844.
- Alkharashi M, Lindsley K, Law HA, et al. Medical interventions for acanthamoeba keratitis. Cochrane Database Syst Rev. Feb 24 2015; 2015(2): Cd010792. doi: 10.1002/14651858.CD010792.pub2.
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. Jan 01 2015; 4: 1. doi: 10.1186/2046-4053-4-1.
- Dart JKG, Papa V, Rama P, et al. The Orphan Drug for Acanthamoeba Keratitis (ODAK) trial: a Phase3 trial of PHMB (polihexanide) 0.08% with placebo versus PHMB 0.02% with propamidine 0.1%. Under review. 2023.
- National Heart Blood and Lung Institute. Study quality assessment tools. Accessed 04/05/2023, https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- Siemieniuk R, Guyatt G. What is GRADE? BMJ. Accessed 30/09/2022, 2022. https://bestpractice.bmj.com/info/toolkit/learn-ebm/what-is-grade/.
- Papa V, Rama P, Radford C, et al. Acanthamoeba keratitis therapy: time to cure and visual outcome analysis for different antiamoebic therapies in 227 cases. Br J Ophthalmol. 04 2020; 104(4): 575-581. doi: 10.1136/bjophthalmol-2019-314485.
- Bagga B, Sharma S, Gour RPS, et al. A randomized masked pilot clinical trial to compare the efficacy of topical 1% voriconazole ophthalmic solution as monotherapy with combination therapy of topical 0.02% polyhexamethylene biguanide and 0.02% chlorhexidine in the treatment of Acanthamoeba keratitis. Eye (Lond). May 2021; 35(5): 1326-1333. doi: 10.1038/s41433-020-1109-4.
- Lim N, Goh D, Bunce C, et al. Comparison of polyhexamethylene biguanide and chlorhexidine as monotherapy agents in the treatment of Acanthamoeba keratitis. Am J Ophthalmol. Jan 2008; 145(1): 130-5. doi: 10.1016/j.ajo.2007.08.040.
- Bonini S, Di Zazzo A, Varacalli G, et al. Acanthamoeba Keratitis: Perspectives for Patients. Curr Eye Res. 06 2021; 46(6): 771-776. doi: 10.1080/02713683.2020.1846753.
- Nasef MH, El Emam SY, ElShorbagy MS, et al. Acanthamoeba Keratitis in Egypt: Characteristics and Treatment Outcomes. Clin Ophthalmol. 2021; 15: 1339-1347. doi: 10.2147/OPTH.S301903.
- Jo YJ, Jang SK, Lee J, et al. A 5-Year Review of Acanthamoeba Keratitis Related to Wearing Contact Lenses in Korea. Eye Contact Lens. Jul 2020; 46(4): 223-227. doi: 10.1097/icl.0000000000000669.
- Megha K, Thakur A, Khurana S, et al. Acanthamoeba keratitis: A 4-year review from a tertiary care hospital in North India. Nepal J Ophthalmol. Jan 2020; 12(23): 83-90. doi: 10.3126/nepjoph.v12i1.24769.
- Musayeva A, Riedl JC, Schuster AK, et al. Topical Voriconazole as Supplemental Treatment for Acanthamoeba Keratitis. Cornea. Aug 2020; 39(8): 986-990. doi: 10.1097/ico.0000000000002315.
- Hassan F, Bhatti A, Desai R, et al. Analysis from a year of increased cases of Acanthamoeba Keratitis in a large teaching hospital in the UK. Cont Lens Anterior Eye. Oct 2019; 42(5): 506-511. doi :10.1016/j.clae.2019.04.009.
- Randag AC, van Rooij J, van Goor AT, et al. The rising incidence of Acanthamoeba keratitis: A 7-year nationwide survey and clinical assessment of risk factors and functional outcomes. PLoS One. 2019; 14(9): e0222092. doi: 10.1371/journal.pone.0222092.
- Carnt N, Robaei D, Minassian DC, et al. Acanthamoeba keratitis in 194 patients: risk factors for bad outcomes and severe inflammatory complications. Br J Ophthalmol. Oct 2018; 102(10): 1431-1435. doi: 10.1136/bjophthalmol-2017-310806.
- Zhong J, Li X, Deng Y, et al. Associated factors, diagnosis and management of Acanthamoeba keratitis in a referral Center in Southern China. BMC Ophthalmol. Oct 02 2017; 17(1): 175. doi: 10.1186/s12886-017-0571-7.
- Jiang C, Sun X, Wang Z, et al. Acanthamoeba keratitis: clinical characteristics and management. Ocul Surf. Apr 2015; 13(2): 164-8. doi: 10.1016/j.jtos.2015.01.002.
- Erdem E, Evcil Y, Yagmur M, et al. Non-contact lens use-related Acanthamoeba keratitis in southern Turkey: evaluation of risk factors and clinical features. Eur J Ophthalmol. Mar-Apr 2014; 24(2): 164-72. doi: 10.5301/ejo.5000357.
- Ku JY, Chan FM, Beckingsale P. Acanthamoeba keratitis cluster: an increase in Acanthamoeba keratitis in Australia. Clin Exp Ophthalmol. Mar 2009; 37(2): 181-90. doi: 10.1111/j.1442-9071.2008.01910.x.
- Lin HC, Hsiao CH, Ma DH, et al. Medical treatment for combined Fusarium and Acanthamoeba keratitis. Acta Ophthalmol. Mar 2009; 87(2): 199-203. doi: 10.1111/j.1755-3768.2008.01192.x.
- Sun X, Zhang Y, Li R, et al. Acanthamoeba keratitis: clinical characteristics and management. Ophthalmology. Mar 2006; 113(3): 412-6. doi: 10.1016/j.ophtha.2005.10.041.
- Pérez-Santonja JJ, Kilvington S, Hughes R, et al. Persistently culture positive acanthamoeba keratitis: in vivo resistance and in vitro sensitivity. Ophthalmology. Aug 2003; 110(8): 1593-600. doi: 10.1016/s0161-6420(03)00481-0.
- Azuara-Blanco A, Sadiq AS, Hussain M, et al. Successful medical treatment of Acanthamoeba keratitis. Int Ophthalmol. 1997; 21(4): 223-7. doi: 10.1023/a:1006055918101.
- Skarin A, Florén I, Kiss K, et al. Acanthamoeba keratitis in the south of Sweden. Acta Ophthalmol Scand. Dec 1996; 74(6): 593-7. doi: 10.1111/j.1600-0420.1996.tb00742.x.
- Elder MJ, Kilvington S, Dart JK. A clinicopathologic study of in vitro sensitivity testing and Acanthamoeba keratitis. Invest Ophthalmol Vis Sci. Mar 1994; 35(3): 1059-64.
- Caruso C, Eletto D, Rinaldi M, et al. Effectiveness and Safety of Topical Chlorhexidine and Vitamin E TPGS in the Treatment of. J Clin Med. Nov 23 2020; 9(11)doi: 10.3390/jcm9113775.
- Bagga B, Joseph J, Garg P, et al. Efficacy of Topical Miltefosine in Patients with Acanthamoeba Keratitis: A Pilot Study. Ophthalmology. 05 2019; 126(5): 768-770. doi: 10.1016/j.ophtha.2018.12.028.
- Kosrirukvongs P, Wanachiwanawin D, Visvesvara GS. Treatment of acanthamoeba keratitis with chlorhexidine. Ophthalmology. Apr 1999;106(4): 798-802. doi: 10.1016/s0161-6420(99)90169-0
- Seal D, Hay J, Kirkness C, et al. Successful medical therapy of Acanthamoeba keratitis with topical chlorhexidine and propamidine. Eye (Lond). 1996;10 ( Pt 4):413-21. doi:10.1038/eye.1996.92.
- Varga JH, Wolf TC, Jensen HG, et al. Combined treatment of Acanthamoeba keratitis with propamidine, neomycin, and polyhexamethylene biguanide. Am J Ophthalmol. Apr 15 1993; 115(4): 466-70. doi: 10.1016/s0002-9394(14)74448-4.
- Hargrave SL, McCulley JP, Husseini Z. Results of a trial of combined propamidine isethionate and neomycin therapy for Acanthamoeba keratitis. Brolene Study Group. Ophthalmology. May 1999; 106(5): 952-7. doi: 10.1016/s0161-6420(99)00515-1.
- Radford CF, Lehmann OJ, Dart JK. Acanthamoeba keratitis: multicentre survey in England 1992-6. National Acanthamoeba Keratitis Study Group. Br J Ophthalmol. Dec 1998; 82(12): 1387-92. doi: 10.1136/bjo.82.12.1387.
- Mathers W. Use of higher medication concentrations in the treatment of acanthamoeba keratitis. Arch Ophthalmol. Jun 2006; 124(6): 923. doi: 10.1001/archopht.124.6.923.
- Donoso R, Mura JJ, López M. [Acanthamoeba keratitis treated with propamidine and polyhexamethyl biguanide (PHMB)]. Rev Med Chil. Apr 2002; 130(4): 396-401. Queratitis por acantoamoeba tratadas con propamidina y polihexametil biguanida (PHMB).
- Parija SC, Prakash MR, Rao VA, et al. Acanthamoeba keratitis in Pondicherry. J Commun Dis. Jun 2001; 33(2): 126-9.
- Bacon AS, Dart JK, Ficker LA, et al. Acanthamoeba keratitis. The value of early diagnosis. Ophthalmology. Aug 1993; 100(8): 1238-43. doi: 10.1016/s0161-6420(93)31499-5.
- Rahimi F, Rafizadeh SM, Beheshtnejad AH, et al. Clinical Outcomes in Acanthamoeba Keratitis Treated with Polyhexamethylene Biguanide as Monotherapy. Journal of Current Ophthalmology. 2014; 26(1): 41-47.
- Revathi R, Rathinam SR, Velayudhan A. Efficacy and Safety of Combination Therapy With Two Biguanides in Higher Concentration in Acanthamoeba Keratitis. American Academy of Ophthalmology. https://secure.aao.org/aao/meeting-archive.
- Jimmy LW, Barkham T, Ming CQ, et al. Reduction in length of hospitalisation for microbial keratitis patients: a prospective study. Int J Health Care Qual Assur. 2009; 22(7): 701-8. doi: 10.1108/09526860910995038.
- Hsu CC, Kuo YS, Lin PY, et al. Overnight orthokeratology-associated Acanthamoeba keratitis at a tertiary referral hospital in Taiwan: A retrospective case-control study. J Chin Med Assoc. Mar 1 2022; 85(3): 381-387. doi: 10.1097/jcma.0000000000000676.
- Wouters KA, Verhoekx JS, van Rooij J, et al. Topical corticosteroids in Acanthamoeba keratitis: Friend or foe? Eur J Ophthalmol. Jan 2022; 32(1): 170-175. doi: 10.1177/1120672120973606.
